Gyros Protein Technologies introduces PurePep EasyClean Auto Kit for automated parallel peptide purification

New automated kit overcomes major bottleneck in peptide production, providing optimized simultaneous workflows
Uppsala, Sweden, 20 July 2023: Gyros Protein Technologies AB, a leading provider of peptide synthesizers and reagents, and a pioneer in automated nanoliter-scale immunoassays, today announced the launch of its PurePep® EasyClean (PEC) Auto Kit for the automated parallel purification of peptides, accelerating downstream processes for research, development, and production of simple to complex peptide drugs and therapies.
Based on Gyros Protein Technologies’ orthogonal PurePep EasyClean technology, the PEC Auto Kit utilizes a simple catch-and-release methodology for purification of chemically synthesised peptides The new Auto Kit allows users of the PurePep Chorus and Symphony® X synthesizer to perform peptide synthesis and purification on a single instrument, further increasing process efficiency.
Conventional chromatography, including reversed-phase liquid chromatography (RP-HPLC) is a major bottleneck in peptide production, both in academia and industry, due to its limited capacity in higher throughput purifications, and the significant volumes of toxic solvents required. In addition, operation of RP-HPLC is labour intensive and requires specialist skills. Gyros Protein Technologies’ PEC Auto Kit requires up to 90% less hands-on time per peptide (6 minutes vs ~ 1 hour) and 90% less solvent consumption, facilitating parallel purification of 6 or more purified peptides in under 6 hours.
Mark Vossenaar, General Manager, Biopharmaceutical Development Division, Gyros Protein Technologies, commented: “The PEC Auto Kit is the first automated solution for parallel peptide purification to remove a major bottleneck in the downstream production process, saving time and resources for this otherwise rate-limiting and labour-intensive step. The first newly developed product in the PurePep family following acquisition of the PEC technology in 2022, the introduction of the PEC Auto Kit further reflects our commitment to providing reliable and efficient solutions for the purification of high-quality peptides for research and production.”
Adrian Glas, CEO, Peptides Speciality Laboratories said: “As a customer, the PurePep EasyClean technology has been an established method for purification in our labs from the very beginning.The introduction of the PEC Auto kit is the missing piece to a fully automated peptide manufacturing process. Now allowing for parallel post-synthesis purification, the new platform was easily integrated into our laboratory methods and has provided immediate benefits. The automation has taken it to the next level in terms of time efficiency and resource reduction!”
For more information on the PurePep® EasyClean Auto Kit, please visit:
www.gyrosproteintechnologies.com/peptides/products/purepep-easyclean-auto-kit
Notes to Editors
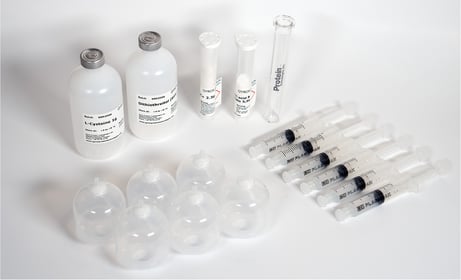
PurePep® EasyClean (PEC) Auto Kit |

Parallel peptide purification on PurePep Chorus using PEC Auto Kit |
For high-resolution image please contact Zyme Communications.
Media Contact:
Dr Ben Rutter
Zyme Communications:
Tel: +44 (0) 7920 770 935
Email: ben.rutter@zymecommunications.com
Mark Vossenaar
Gyros Protein Technologies
Tel: +31 6 34735894
Email: mark.vossenaar@gyrosproteintech.com
About Gyros Protein Technologies
Gyros Protein Technologies enables bioanalytical solutions and peptide synthesis, helping scientists increase biomolecule performance and productivity in research, drug discovery, pre-clinical and clinical development, and bioprocess applications. Proprietary high performance nanoliter-scale immunoassay platforms, Gyrolab® xPand, and Gyrolab xPlore™, and Gyrolab immunoassay kits are used by scientists at leading pharmaceutical, biotech, CRO, and CMO companies in the development and manufacturing of various biotherapies including those based on peptides, antibodies, cell and gene therapies and vaccines. Gyrolab immunoassays provide key workflow advantages of speed, automation, and low reagent usage, and assay quality advantages of wide dynamic range with robust precision and reproducibility in applications including pharmacokinetics/pharmacodynamics, immunogenicity, and analysis of bioprocess-related impurities. Our low to mid-scale peptide synthesizer platforms, PurePep® Chorus, Symphony® X, and PurePep® Sonata+, and chemistries deliver uncompromising purity, flexibility, and quality synthesis for discovery and pre-clinical studies of simple to complex multifunctional peptides. Our peptide synthesis and bioanalytical solutions accelerate the discovery, development, and manufacturing of safer biotherapeutics. Gyros Protein Technologies is part of the Biopharmaceutical Development Division of Mesa Laboratories, Inc. (Nasdaq: MLAB).
Forward Looking Statements
This release contains “forward-looking statements” – that is, statements that relate to future, not past, events. In this context, forward-looking statements often address our expected future business and financial performance and financial condition, and often contain words such as “expect,” “assume,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “see,” or “will.” Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. These risks and uncertainties include the duration and severity of the COVID-19 pandemic, expectations regarding our products, our ability to develop and market new or improved products, our ability to compete effectively, international legal and regulatory risks, and product quality and liability issues. For further information regarding our risks and uncertainties, please refer to the “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operation” in Mesa Laboratories, Inc.’s public reports filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q. We caution you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. We disclaim any obligation to update these forward-looking statements.