Matching microsampling with miniaturized bioanalysis is revolutionizing pharma biotherapeutic development
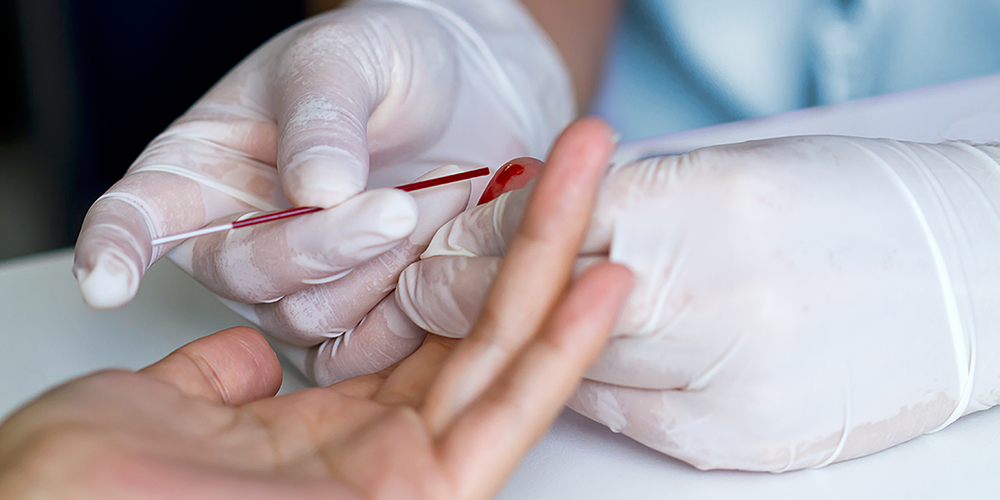
Microsampling is revolutionizing sample collection for preclinical and clinical research and diagnostics. It is enabling significant reductions in animal use and creating new possibilities for at-home, patient-centric sampling that will have a major impact on how clinical trials will be run in the future. A key factor to success is matching microsampling methods with bioanalytical techniques such as immunoassays that enable reliable, sensitive detection of analytes in small volumes of complex matrix.
Revisiting microsampling and the 3Rs
In 2014 we published a couple of blog articles highlighting the value of microsampling in addressing the 3Rs (Replace, Reduce, Refine) effort to minimize animal use in toxicological and pharmacological testing. One microsampling technique, capillary microsampling (CMS), proved to be a reliable method that could deliver microliter-scale blood samples that made serial sampling of individual mice possible. Combining CMS and sample analysis using Gyrolab system meant that a pharmacokinetic (PK) profile for a biotherapeutic could be generated from a single animal (Joyce et al, 2014). Serial sampling of individual animals not only reduced the number of animals used in nonclinical studies but also reduced the variability in data caused by biological variation, for example when sampling satellite animals in parallel to generate individual data points. At the time, AstraZeneca, for example, showed that incorporating microsampling into a preclinical study workflow, together with other improvements in methods, project coordination and study design led to a 50% reduction in animal use (Törnqvist et al, 2014).
Microsampling clearly offers a lot of advantages but there is still some resistance in the industry to a transition from conventional methods, and a perceived reluctance by regulatory authorities to accept data generated using microsampling. The regulatory landscape is changing, however, and microsampling is poised to become a standard method to generate samples in nonclinical/preclinical research (ref e.g., surveys referred to in white paper).
COVID-19: a paradigm shift in health care
My interest in microsampling recently took a sharp turn from nonclinical work to clinical research and diagnostics because of the COVID-19 pandemic.
A very simple microsampling method, dried blood spot (DBS), had already been used since the 1960s to screen babies for phenylketonuria and to monitor dietary treatment. DBS has also been used in many studies, both clinical and preclinical, but there are issues with the method, such as the effect hematocrit can have on results (Fan and Lee, 2012). With necessity being the mother of invention, the increasing need for simple, relatively painless self-sampling techniques to accommodate COVID-related isolation has driven development of other techniques such as volumetric absorptive microsampling (VAMS). The possibilities offered by these developments came to a head early in the pandemic with a desperate need for samples from the population to get some insight into the spread of the virus and the development of immune response.
Microsampling and home-based self-testing offers a solution to the problems of having to visit a clinic, risk contact with others, and deliver an unnecessarily large blood sample. A patient can instead use a microsampling device to self-sample, put the sample in the post, and get the results electronically or by mail. For example, home-based microsampling was used early in the pandemic to determine the levels of SARS-CoV-2 antibodies in those with no confirmed history of COVID-19 in the US.
The result has been a shift of patient-centric (micro)sampling using relatively painless techniques from limited groups such as diabetics to the general population. As I discovered, this will have enormous effects not only on home-based sampling for diagnosis but also on how clinical trials are run.
Apart from the devastating death toll, COVID-19 has had an incredible impact on society. For example, the need for social distancing has led to new behavioral patterns. Whenever possible we have worked from home, ordered consumer goods on-line, held meetings by video conferencing. And there is a good chance that a lot of these changes are here to stay. The pandemic has also opened the public’s eyes to the possibilities with routine clinical sampling, from home-based ‘self-testing’ to being sampled at a drive thru.
Microsampling as a key component of future clinical trials
The COVID-19 pandemic has disrupted clinical trials and highlighted the limitations of the current approach (Anderson et al, 2021). This has resulted in efforts to develop a de-centralized clinical trial model involving patient-centric testing using microsampling methods such as VAMS, which has already been used in many clinical trials (Harahap et al, 2020). This approach may well find broader acceptance in a post-COVID-19 world that recognizes the need for rapid population sampling to fight infectious disease.
Merck (known as MSD outside the U.S. and Canada), a major pharmaceutical company with a worldwide presence, has reported plans for a digitally enabled clinical trials initiative aimed at shifting clinical trials from site-centric to patient-centric testing (Dockendorf et al, 2021). This includes at-home sampling, for example using DBS or VAMS, together with technologies to monitor and improve patient adherence (smart dosing), and digital devices to collect physiological data. In other words, a true paradigm shift in how clinical trials will be run.
Matching microsampling and bioanalysis
Developments in bioanalytical methods have obviously been a key driver in making microsampling a practical alternative in both preclinical and clinical work. Matching the bioanalytical method with the microsample is key to success. Bioanalysis must be able to deliver reliable data from small amounts of analyte in complex matrices such as blood or saliva. With its nanoliter-scale flow-through technology, Gyrolab system is an immunoassay platform that has proved its value in several studies based on microsampling.
Find out more by downloading the White Paper:
In this white paper, we describe microsampling methods, how they are being used in nonclinical/preclinical and clinical studies and diagnostics, how the biopharmaceutical industry and regulatory authorities are reaching common ground to make their application widespread, and the demands microsampling place on immunoassays (ligand-binding assay, LBA). You can also find out how Gyrolab system is enabling the analysis of a range of analytes in microsamples of complex matrices.
References:
Anderson M. How the COVID-19 pandemic is changing clinical trial conduct and driving innovation in bioanalysis. Bioanalysis. 2021 Aug;13(15):1195-1203. doi: 10.4155/bio-2021-0107. Epub 2021 Jul 19. PMID: 34275327; PMCID: PMC8288280.
Dockendorf MF, et al. (2021), Digitally Enabled, Patient-Centric Clinical Trials: Shifting the Drug Development Paradigm. Clin Transl Sci, 14: 445-459.
Harahap Y, et al. Volumetric Absorptive Microsampling as a Sampling Alternative in Clinical Trials and Therapeutic Drug Monitoring During the COVID-19 Pandemic: A Review. Drug Des Devel Ther. 2020 Dec 31;14:5757-5771. doi: 10.2147/DDDT.S278892. PMID: 33414636; PMCID: PMC7783192.
Joyce, AP et al. One mouse, one pharmacokinetic profile: quantitative whole blood serial sampling for biotherapeutics. Pharm Res 2014 Jul;31(7):1823-33. doi: 10.1007/s11095-013-1286-y.
Li F, et al. Perforated dried blood spot accurate microsampling: the concept and its applications in toxicokinetic sample collection. J Mass Spectrom. 2012 May;47(5):655-67. doi: 10.1002/jms.3015.
Törnqvist E, et al. Strategic Focus on 3R Principles Reveals Major Reductions in the Use of Animals in Pharmaceutical Toxicity Testing. PLoS ONE 2014 9(7): e101638.