Putting a new spin on immunoassays for gene therapy – Part 1
How to detect vector immunogenicity 24× faster and with 10× less sample
Cell and gene therapy promises to revolutionize medicine by correcting underlying genetic causes of disease or conferring new functions to cells to combat disease and has already seen spectacular successes in health care, ranging from curing rare eye diseases, to the treatment of sickle cell disease and a number of cancers. Immunoassays can be important analytical tools in cell and gene therapy and the careful choice of an immunoassay platform can improve productivity by boosting workflows, data quality and greatly reducing the consumption of precious samples, for example in detecting antibodies against lentivirus vectors that are commonly used in cell and gene therapy.
The search for a better immunoassay platform to monitor antibody response to viral vectors
Oxford Biomedica has established itself on the gene therapy stage, being the first to administer lentiviral vectors in vivo and as the first lentiviral vector containing product to gain approval for advanced therapy in the US using a unique LentiVector® enabled technology (Novartis Kymriah®). Over 500 patients have been treated with gene therapeutics by Oxford Biomedica or its partners, and a number of new therapies are in development.
Lentiviral vectors can be used ex vivo, for example in the preparation of CAR (chimeric antigen receptor) T cells for the treatment of acute lymphoblastic leukaemia with Kymriah. In vivo use includes treatment of Parkinson’s disease and retinal diseases.
The LentiVector® delivery platform developed at Oxford Biomedica uses third-generation ‘minimal’ lentiviral vectors based on the equine infectious anemia virus (EIAV) and human immunodeficiency virus-1 (HIV-1). Viral vectors contain a number of potential antigens, and immune responses against these are monitored in patients using semi-quantitative ELISA to determine relative units of lentiviral vector-associated antibodies in peripheral blood serum. Antibody responses against a lysate of cells that were transfected with lentiviral vector were compared to those against non-transfected cell lysates as a control. The laborious and time-consuming immunoassay included a number of steps:
- coating a plate with cell lysate,
- adding the test sera/standards/QCs/negative control,
- adding anti-human IgG HRP-conjugated secondary antibody, and
- measuring using a chromogenic substrate.
The team at Oxford Biomedica decided that they needed an improved immunoassay method that would give more rapid reproducible results, facilitate automation to free up a scientist’s time, and one that would efficiently handle small sample volumes.
How to detect lentiviral vector immunogenicity 24X faster and with 10X less sample
At the latest European Gyrolab User Meeting held in Milan in June 2019, Dr. Daniel Blount, Principal Scientist at Oxford Biomedica, presented why the assay development team chose Gyrolab® xPlore™ to measure the immunogenicity of lentiviral vectors, and some results from the initial method development.
The team at Oxford Biomedica saw that by using the Gyrolab system they would be able to run an assay in 90 minutes compared to 36 hours using ELISA. Assay development time could also be expected to be much shorter, and the automation would mean more free time for the scientist. Running a Gyrolab assay would also be much less laborious than an ELISA, which reduced the need to stockpile samples for a large batch run. They also noted the advantage of being able to run analyses with 10× less sample volume than was required for the ELISA, and the low matrix interference that reduces the need for dilution, which increases sensitivity.
The aim at Oxford Biomedica is to use Gyrolab system to develop assays to detect antibodies specific to a number of lentiviral antigens, including VSV-G, EIAV p26 protein, neomycin phosphotransferase II (a selection protein), and transgene proteins. (Fig. 1).
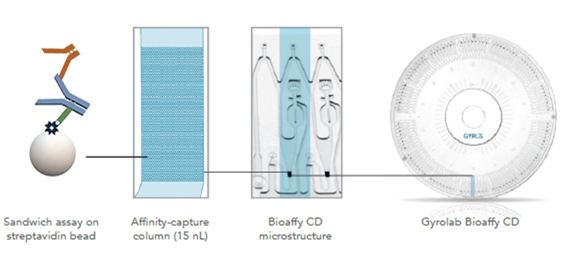
Figure 1. Assay design for detecting antibodies against the lentivirus envelope protein VSV-G
Assay development is at an early phase, and has included studying the effects of a number of parameters, such as choice of Gyrolab CD, design and concentration of capture and detection reagents, and dilution buffers.
This application is just one example of how Gyrolab systems can simplify and accelerate the development and evaluation of cell and gene therapy products. In the next article in this series, we will be looking at how Gyrolab system has been used to determine the potency of a transgene that encodes an IgG antibody.
You can find out more about the benefits of using Gyrolab systems in cell and gene therapy by learning about the new Gyrolab p24 Kit.