Host Cell Protein (HCP) Impurities in Biotherapeutic Drug Development and Manufacturing
Host Cell Protein (HCP) Impurities
Cell lines are essential for developing and manufacturing biopharmaceuticals and viral vectors, but all cell line expression results in contamination by residual host cell proteins (HCPs). HCPs may be released into the culture medium during expression—through secretion or cell lysis during harvest (Luo et al.)—and optimization of expression conditions (cell media, harvest time, temperature) and subsequent purification and polishing steps will reduce HCP levels in the manufactured material. If HCP clearance fails, leading to excessive levels in the final product, it can have extremely costly effects, including delayed clinical trial starts and halted manufacturing (Fischer et al. 2017, Vanderlaan et al. 2018).
HCP impurities may cause toxicity and unwanted immune responses in patients, so it's essential—both from a regulatory and patient safety perspective—to ensure these have been cleared in the final product. The risk of immune response is the main concern, but less acute effects caused by biologically or enzymatically active HCPs with potential to degrade the drug or other components of the drug formulation also need consideration. Reduced drug stability caused by proteolytic enzymes will lead to lower efficacy and may result in potentially harmful levels of degradation products.
HCP Guidelines
 Regulatory bodies like the U.S. Food and Drug Administration (FDA) require that HCP levels are measured and kept at acceptable levels throughout production stages and in the drug product. Acceptable limits for HCP content in products for human use are guided by precedence (Bracewell 2015), and most biotechnology products reviewed and approved by the FDA have HCP levels of <100 ng/mL (Bracewell et al. 2015, Chon and Zarbis-Papastoitsis 2011, Hogwood et al. 2014). To keep HCP impurities to a minimum in the final product, their clearance must be monitored in each process optimization step—from expression to purification and polishing.
Regulatory bodies like the U.S. Food and Drug Administration (FDA) require that HCP levels are measured and kept at acceptable levels throughout production stages and in the drug product. Acceptable limits for HCP content in products for human use are guided by precedence (Bracewell 2015), and most biotechnology products reviewed and approved by the FDA have HCP levels of <100 ng/mL (Bracewell et al. 2015, Chon and Zarbis-Papastoitsis 2011, Hogwood et al. 2014). To keep HCP impurities to a minimum in the final product, their clearance must be monitored in each process optimization step—from expression to purification and polishing.
The BioPhorum Development Group (BPDG), with representatives from 26 companies, published a shared view on high-risk HCPs (Jones et al. 2021), focusing on biopharmaceuticals produced in CHO cells and purified by protein A affinity chromatography—a widely used, robust purification step for mAbs. The importance of monitoring throughout the process was highlighted, as some HCPs escape clearance in upstream purification, either by binding to the product (Clavier 2020) or interacting with the protein A column (Levy et al. 2014). The team concluded by listing recommendations for minimizing HCP content during early manufacturing steps to avoid repercussions closer to delivery of the final product (Jones et al. 2021). HCP detection assays therefore need to measure varying concentrations of HCPs in samples ranging from cell lysates to purified product with acceptable throughput.
HCP Detection and Quantification
HCP Detection Assay Requirements
 Since optimization of expression conditions, lysis, and each purification step aims to maximize production and minimize contaminants, it's essential to accurately measure HCP levels at each production step and in the final product. The assays used must be suited for measuring a broad range of HCP concentrations in various sample types, ranging from cell lysates to purified product. The assay also needs to be robust with adequate throughput. In addition, to ensure continuous support in development and manufacturing without interruptions, the assay needs to be run by different operators—ideally at different locations—with reproducible results.
Since optimization of expression conditions, lysis, and each purification step aims to maximize production and minimize contaminants, it's essential to accurately measure HCP levels at each production step and in the final product. The assays used must be suited for measuring a broad range of HCP concentrations in various sample types, ranging from cell lysates to purified product. The assay also needs to be robust with adequate throughput. In addition, to ensure continuous support in development and manufacturing without interruptions, the assay needs to be run by different operators—ideally at different locations—with reproducible results.
HCP Testing by ELISA
The gold standard for measuring HCP levels is Enzyme-Linked Immunosorbent Assays (ELISAs) based on polyclonal antibodies against HCP antigens from various expression organisms. However, traditional plate-based ELISA requires significant manual handling and analysis time and may not meet the throughput needed by process development teams. Also, ELISAs have a relatively narrow dynamic range, requiring sample dilution to keep HCP concentrations within the analytical window of the assay. This increases the risk of mistakes and the need for retesting, which wastes material and delays delivery timelines (Petrovic et al. 2019). Automated ELISAs can improve throughput, but the dynamic range remains a major limitation. The validated HCP methods for product release are typically run by ELISA, but upstream and downstream process development and optimization frequently use more sensitive methods with higher throughput.
HCP Testing by Gyrolab
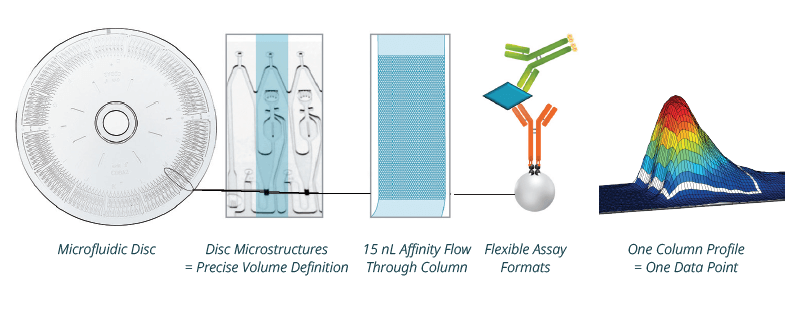
An alternative immunoassay technology for HCP measurements is the automated Gyrolab platform. The platform is well recognized for use in bioanalytical PK and toxicology studies, with increasing use for titer and impurity measurement in process development (Petrovic et al. 2019, Voigtman and Foettinger-Vacha 2023). The Gyrolab technology uses a proprietary disc with well-defined volume structures (20 nL–4 μL) and miniaturized affinity columns (15 nL) coated with streptavidin beads to which biotinylated capture reagents can be attached. Like ELISA, the Gyrolab method of measuring HCPs employs polyclonal anti-HCP antibodies for capture and detection.
HCP testing by Gyrolab offers a broad concentration range, minimizing the need for sample dilutions, and it tolerates a wide selection of biological samples. The automated format minimizes manual pipetting steps, enabling reproducible results even when handled by different operators—making it a reliable tool for HCP detection. For these reasons, several companies use Gyrolab as their go-to method for HCP testing in cell line and process development and for batch release testing (Petrovic et al. 2019, Voigtman and Foettinger-Vacha 2023).
The Gyrolab Platform
 Microfluidic immunoassay technology with automated delivery of samples and reagents by centrifugal force and capillary action. Analytes are captured by biotinylated reagents on streptavidin beads in flow-through 15 nL-columns on discs and typically detected by Alexa Fluor™ labeled detection agents.
Microfluidic immunoassay technology with automated delivery of samples and reagents by centrifugal force and capillary action. Analytes are captured by biotinylated reagents on streptavidin beads in flow-through 15 nL-columns on discs and typically detected by Alexa Fluor™ labeled detection agents.
References
- Bracewell DG, Francis R & Smales CM (2015) The Future of Host Cell Protein (HCP) identification during process development and manufacturing linked to a risk-based management for their control. Biotechnology and Bioengineering 112:1727-1737
- Clavier S, Fougeron D, Petrovic S, Elmaleh H, Fourneaux C, Bugnazet D, Duffieux F, Masiero A, Mitra-Kaushik S, Genet B, Fromentin Y, Kreiss P, Laborderie B, Brault D, and Menet JM (2020) Improving the analytical toolbox to investigate copurifying host cell protein presence: N-(4)-(beta-acetylglucosaminyl)-l-asparaginase case study. Biotechnology and Bioengineering 117:3368-3378
- Chon JH and Zarbis-Papastoitsis G (2011) Advances in the production and downstream processing of antibodies. New Biotechnology 28:458-463
- Fischer SK, Cheu M, Peng K, Lowe J, Araujo J, Murray E, Mcclintock D, Matthews J, Siguenza P, and Song A (2017) Specific immune response to phospholipase B-like 2 protein, a host cell impurity in Lebrikizumab material AAPS journal 19(1):254-263
- Hogwood CEM, Bracewell DG, Smales CM (2014) Measurement and control of host cell proteins (HCPs) in CHO cell bioprocesses. Current Opinion in Biotechnology 30:153-160
- Jones M, Palackal N, Wang F, Gaza-Bulseco G, Hurkmans K, Zhao Y, Chitikila C, Clavier S, Liu S, Menesale E, Schonenbach NS, Sharma S, Valax P, Waerner T, Zhang L & Connolly T (2021) "High-risk" host cell proteins (HCPs): A multi-company collaborative view. Biotechnology and Bioengineering 118:2870-2885
- Levy NE, Valente KN, Choe LH, Lee KH, and Lenhoff AM (2014) Identification and characterization of host cell protein product-associated impurities in monoclonal antibody processing. Biotechnology and Bioengineering 111(5): 904-912
- Luo H, Tie L, Cao M, Hunter AK, Pabst TM, Du J, Field R, Li Y, Wang WK (2019) Cathepsin L Causes Proteolytic Cleavage of Chinese-Hamster-Ovary Cell Expressed Proteins During Processing and Storage: Identification, Characterization, and Mitigation. Biotechnology Progress 35(1)
- Petrovic S, Fromentin Y, Pelluet P, Nombret J, Jegat M, Dubois H, Borges L, Kreiss P, Laborderie B, Hincapie M, Kirsch M, Karetsky L, Unger S, Schoch A & Duhau L (2019) HCP analysis to support downstream process development. BioProcess International 17(11-12)
- Vanderlaan M, Zhu-Shimoni J, Lin S, Gunawan F, Waerner T, and Van Kott KE (2018) Experience with host cell protein impurities in biopharmaceuticals. Biotechnology Progress 34(4): 828-837
- Walsh G and Walsh E (2022) Biopharmaceutical benchmarks 2022. Nature Biotechnology 40:1722-1760
- Wurm MJ and Wurm FM (2021) Naming CHO cells for bio-manufacturing: Genome plasticity and variant phenotypes of cell populations in bioreactors question the relevance of old names. Biotechnology Journal 16:2100165