A new spin on ultra-high biomolecular affinity determination
Development of high-affinity binding interactions and utilization of the appropriate tools for affinity determination are the ultimate goals of antibody therapeutic R&D. The expansion of protein engineering methods has provided powerful techniques for affinity maturation of biotherapeutics to accomplish at least part of this goal. In particular, the recent use of phage display methods for antibody affinity evolution have produced high affinity antibodies in the picomolar dissociation constant (KD) range, also with high specificity, such as the TNF-α inhibitor Humira® (AbbVie Inc.)1,2
The successful development of high-affinity, high-specificity antibody therapeutics using these techniques has contributed to the rapid growth of the pipeline for therapeutic antibodies. One glaring roadblock has been that methodologies for affinity determination has lagged behind the antibody engineering advancements, creating the conundrum of having the tools to engineer high-affinity interactions that cannot be effectively measured.
A variety of methods have traditionally been used to measure equilibrium dissociation constants including radioligand binding assays, fluorescence energy resonance transfer (FRET), isothermal titration calorimetry (ITC), surface plasmon resonance (SPR) and Bio-Layer Interferometry (BLI). Of these methods, SPR and BLI are the most commonly used, and while they are label-free their disadvantages include the tethering of one interactant to a solid surface, and they also lack the sensitivity to determine sub-nanomolar affinities or interactions with slow dissociation rates.
This disconnect of protein engineering approaches speeding ahead of affinity measurement techniques is illustrated in a recent publication by Dai et al,3 where a human PD-L1 binding Adnectin™, currently in clinical studies as a positron emission tomography (PET) imaging agent, was engineered to have sub-nanomolar affinity, but the KD could only be estimated by SPR techniques since it was at the detection limits for this technique.
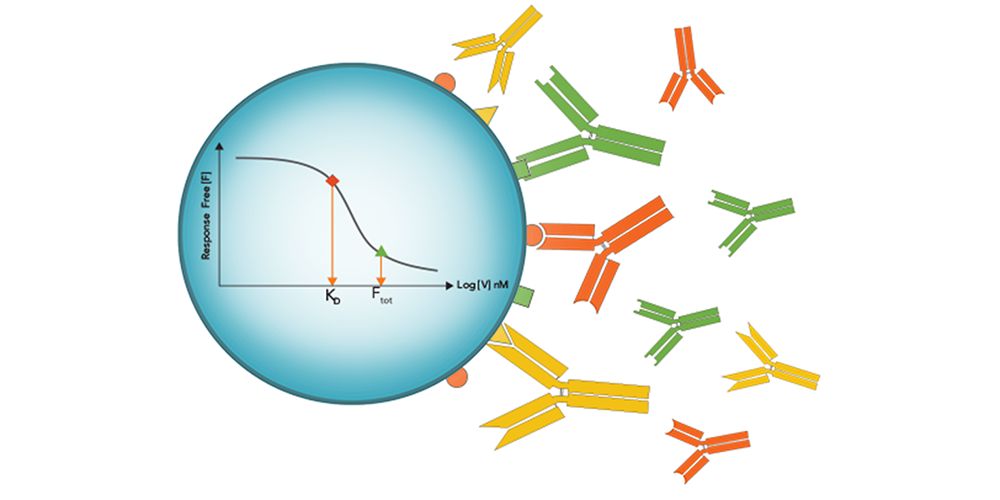
The authors turned to a sensitive immunoassay method where free interactant is measured untethered: in-solution affinity determination with Gyrolab® microfluidic, flow-through affinity column format immunoassays. In this format eliminating incubation times characteristic of plate-based methods or distortions from immobilized surface-based methods, free interactant can be determined with high sensitivity without the equilibrium shift that can result from prolonged incubation or surface washout times.
Gyrolab affinity software module multi-curve analysis allowed the accurate measurement of the affinity Adnectin to human PD-L1 as 20 pM, finally bringing a real KD value to the high-affinity ligand-binding interaction.
These results, in the authors words, "...demonstrated advantages of the miniaturized Gyrolab immunoassay platform to perform thorough yet rapid assay development.” They also noted the new multi-curve fit feature of the Gyrolab solution affinity software is an important upgrade that allows global fitting of affinity curves at different fixed interactant concentrations, yielding a reliable and more accurate KD value.
The unique high-sensitivity and straightforward assay setup of the affinity measurement using Gyrolab solutions opens the door to discovering true affinity measurements of previously incompletely characterized biomolecular interactions and antibody-ligand binding pairs, now enabling affinity measurements to keep up with protein engineering efforts.
Affinity measurements using Gyrolab immunoassays have other advantages including low sample volume, measurements in biological matrices, minimal hands-on time, high reproducibility, and rapid time to results, all pointing towards a bright future for the utilization of this technology for affinity determinations during antibody therapeutic development and production.
References:
- Alfaleh, M A, Alsaab, H O, Mahmoud, A B, et al. (2020). Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. In Frontiers in Immunology 11,1986.
- Lu, R M, Hwang, Y C, Liu, I J, et al. (2020). Development of therapeutic antibodies for the treatment of diseases. In Journal of Biomedical Science 27(1), 1–30.
- Dai, Z, Juneja, J, Schneeweis, L, et al. (2020). Application of the Gyrolab microfluidic platform to measure picomolar affinity of a PD-L1-binding AdnectinTM radioligand for positron emission tomography. BioTechniques, 69(3), 200–205.