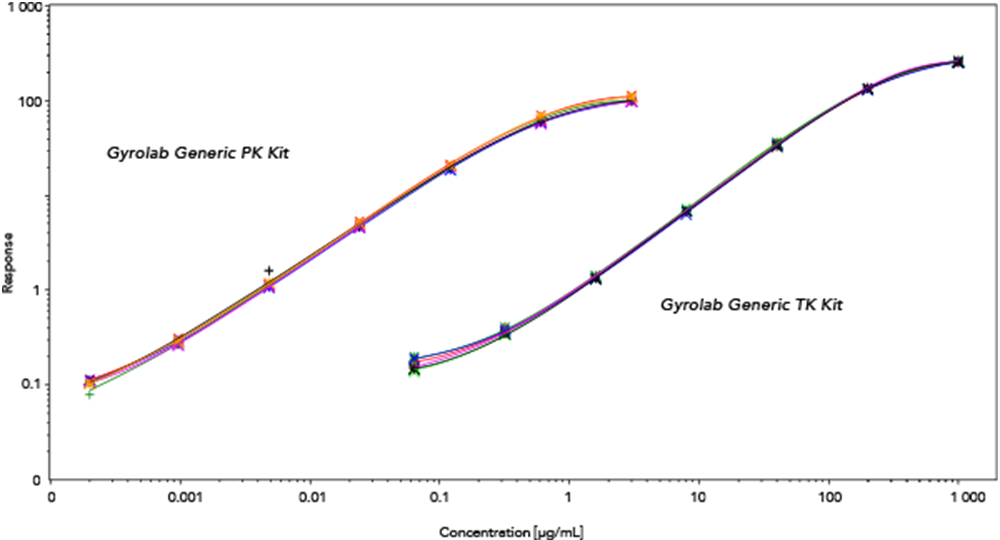
Products
Services
Applications
Resources
User Zone
Careers
Products
Peptide Synthesizers
Find a Product
View All Products
Services
Applications
Resources
About Us
Blog