Introduction
Peptide-related impurities can significantly influence the outcome of drug research and development (e.g.,false-positive results) and must be removed to ensure high assay reliability. Ultimately, such impurities must be removed entirely from active pharmaceutical ingredients to provide the highest quality clinical-grade material.
Purification of chemically synthesized peptides is mainly carried out by traditional chromatographic methods. Reversed-phase high-pressure liquid chromatography (RP-HPLC) is the most commonly used method. Also, reversed-phase flash chromatography (flash) represents an established technique for research-grade peptide purifications and intermediate/clean-up purification steps of higher-grade material. Due to similar properties, however, co-eluting impurities may be overlooked with both these chromatographic techniques.
In contrast, chemo-selective separation principles drive Gyros Protein Technologies orthogonal purification PurePep EasyClean technology. Capping during solid-phase peptide synthesis (SPPS) ensures that only the full-length peptide is accessible for modification with a traceless cleavable purification linker (PEC-Linker) at the end of SPPS. The target peptide can then be isolated from a complex mixture via orthogonal catch-and-release on modified beads.[1-2]
Animation of PurePep EasyClean orthogonal purification technology
In this case study, we demonstrate the orthogonality of PEC with RP-HPLC by purifying the polar fragment of the Histone H3 (1-20) peptide using the Starter Kit.
| TABLE 1: PEPTIDE USED IN THIS STUDY. |
| name |
sequence |
| Histone H3 (1-20) |
H-ARTKQ TARKS TGGKA PRKQL-OH |
Discussion
PEC-grade peptides. The UV-chromatogram of the crude Histone H3 (1-20) appears very clean at first glance (Figure 1, left). However, several capped deletion sequences assemble in the mass analysis of the corresponding peak (inset of Figure 1, left). Major impurities were Ala (A)-, Arg (R)-, and Thr (T) deletions. An analytical HPLC run with 0.1% HFBA resolved the underlying peaks, and a crude purity of 29% was determined (chromatogram is not shown).
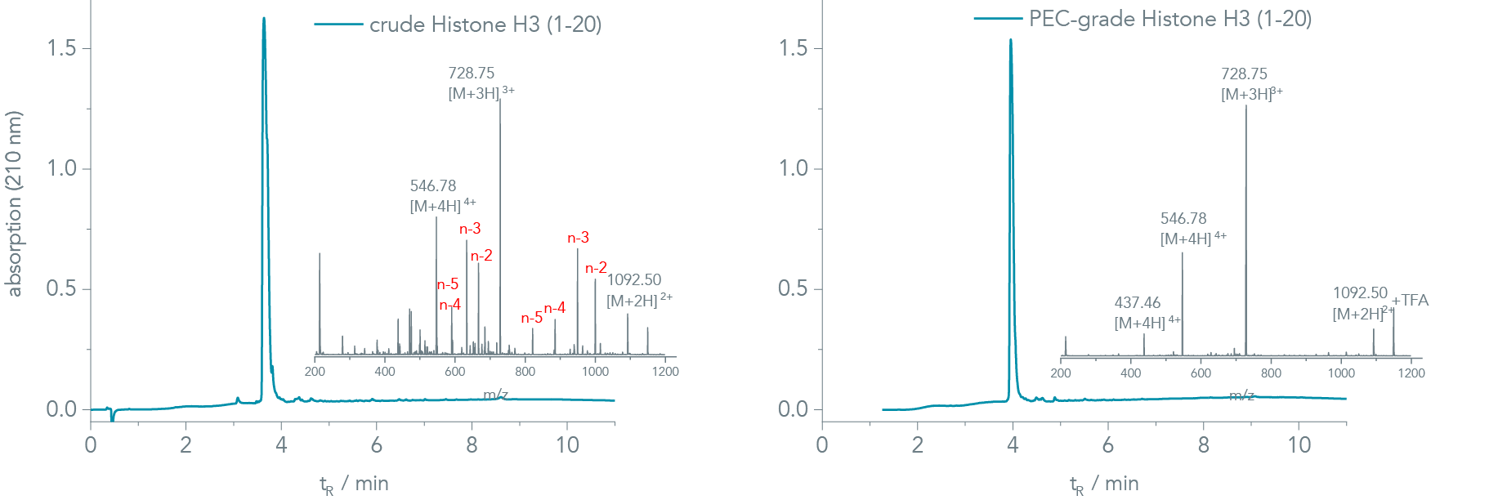
Figure 1 (right) shows the chromatogram of the PEC-purified peptide. A closer look into the mass spectra (insets in Figure 1, right) reveals that PEC removes the co-eluting impurities efficiently in a single step ensuring high assay reliability.
Due to the ability of HFBA to separate the target peptide from the truncated sequences, HFBA-enhanced reversed-phase flash chromatography served as the first-dimension chromatographic purification for comparison. The purity increased to 66% with the applied method (Table 2). On the contrary, a single run with orthogonal PEC resulted in 86% PEC-grade purity.
Clinical grade peptides with PEC. Next, we performed an additional RP-HPLC purification with both the flash- and the PEC-grade peptides as the second dimension purification to obtain clinical-grade peptides. The purities were increased from 29% (crude) to 85% and 96%, respectively (Table 2), clearly demonstrating the superior purification efficiency of PEC in orthogonal combinations with chromatography for peptides that show co-eluting impurities in the crude mixture.
|
TABLE 2: PURITIES AND ACN/TOTAL WASTE USAGE.
|
1st dimension
purification |
2nd dimension
purification |
final
purity |
ACN used |
total waste |
| PEC |
- |
86% |
50 mL |
200 mL |
| PEC |
RP-HPLC |
96% |
1050 mL |
3200 mL |
| Flash |
- |
66% |
500 mL |
1500 mL |
| Flash |
RP-HPLC |
85% |
1500 mL |
4500 mL |
Solvent economy. Chromatographic separation consumes large amounts of organic solvents. Therefore, reducing solvent use - one of the 12 principles of green chemistry - is key to improving the ecological footprint of peptide purification
ACN consumption is a reasonable measure to evaluate the ecological benefit of the orthogonal approach. Due to PEC‘s catch-and-release principles, users work with a small amount of organic solvents and produce less waste. Only 50 mL of ACN and 200 mL of total waste accumulated during the process.
In contrast, the flash purification required 500 mL MeCN and produced 1500 mL total solvent resulting in an overall saving of more than 85% of solvent use and waste.
When considering the additional RP-HPLC purification run, added1000 mL of ACN and 3000 mL of total waste were added.
An orthogonal PEC purification in combination with RP-HPLC results in a total saving of costly solvents and harmful waste of approx. 30%.
Results at a glance
- efficiently remove co-eluting truncations in a single orthogonal purification step
- get the highest purities for challenging peptides with a combination of orthogonal PEC and RP-HPLC
- save up to 85% of solvent use and total waste with orthogonal PEC purification