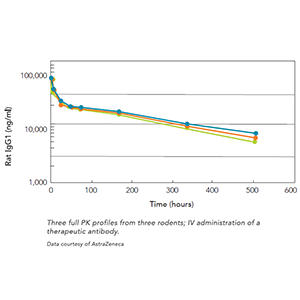
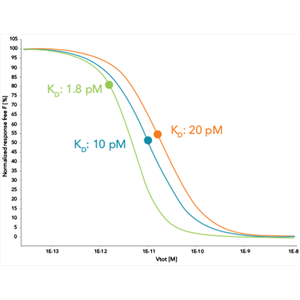
Products
Find a Product
View All Products
Services
Applications
Resources
User Zone
About Gyrolab